Product Specification
ActiveIngredient
L-Histidine, Sodium Chloride, Poloxamer 188, & Hydrochloric Acid
Shelf life
36 months from the date of manufacture
Storage
the unopened lyophilized vial should be stored in a freezer (below -5°C or refrigerator(2~8°C)

Appearance
A thin, transparent circled powder layer that is very well vacuumed at the bottom of the bottle.
Indication
The temporary improvement in the appearance of moderate to severe glabellar lines.
Dosage
100 Units/vial
Highlight
DNA sequence registered in NCBI GeneBank database
World class quality system GMP manufacturing system certified by US FDA, TGA, PMDA, AIFA, ANVISA, COFEPRIS, WHO, etc.
vacuum-dried powder tech
It minimizes denaturalization such as foam or molecular breakage when diluted
One-off botanic toxin with non-animal process
JTOK is made with the usage of the most modern technologies, making it highly
effective toxin with the purity ratio of ≤ 99.9%.

High stability with advance purification process
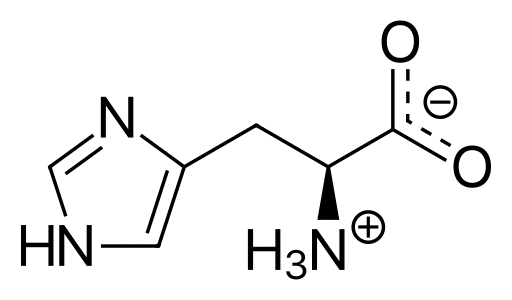
What is Histidine?
Histidine is an essential-amino acid that is
used in the biosynthesis of proteins.
Initially thought essential only for infants, it
has now been shown in longer-term studies
to be essential for adults
Special Active Ingredient L’Histidine
CKD Bio Established in 1941 Top3 Pharmaceutical company in South Korea
GMP CERTIFICATION
We are always following & complying with the latest global GMP standard. After being certified by Korean FDA for BGMP in 1999, we have been obtaining various MOHs’ GMP certifications.
Licensing Status STATE OF THE ART TECHNOLOGY
CKD BiO has been recognized across the world by manufacturing & supplying finest quality APIs & Intermediates (including antibiotics, β-lactamase inhibitor, antidiabetic, immunosuppressant) to the Globe.